Zomebazam
Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| Chemical and physical data | |
| Formula | C15H16N4O2 |
| Molar mass | 284.319 g·mol−1 |
| 3D model (JSmol) |
|
| |
InChI
| |
 N N Y (what is this?) (verify) Y (what is this?) (verify) | |
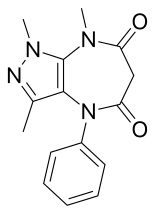
Zomebazam[1] produced by Hoechst is a pyrazolodiazepinone derivative drug with anxiolytic properties. It is structurally related to razobazam and zometapine.[2]
Synthesis
The catalytic hydrogenation of N,2,5-trimethyl-4-phenyldiazenylpyrazol-3-amine[a] (1) over Raney nickel gives 4-amino-1,3-dimethyl-5-methylaminopyrazole[b] (2). Treatment with methyl malonyl chloride[c] (3) gives 4-α-ethoxycarbonylacetylamino-1,3-dimethyl-5-methylaminopyrazole[d] (4). Base-catalyzed lactamization gives (5). The Goldberg reaction completes the synthesis of zomebazam (6).[3][4]

See also
- Benzodiazepine
References
- ^ US 3558605, "4-Aryl-5,6,7,8-tetrahydropyrazolo(3,4-B)-(1,5)diazepine-1H,4H-5,7-diones and medicaments containing same"
- ^ "Zomebazam". psychotropics.dk. 2003. Retrieved 7 December 2009.
- ^ Renger B (1985). "Direkte N-Arylierung von Amiden: Eine Verbesserung der Goldberg-Reaktion". Synthesis. 1985 (9): 856–560. doi:10.1055/s-1985-31364. S2CID 93397774.
- ^ US 4302468, Rackur G, Hoffmann I, issued 1981, assigned to Hoechst Aktiengesellschaft
Notes
- v
- t
- e
Benzodiazepines
- 2-Oxoquazepam
- 3-Hydroxyphenazepam
- Bromazepam
- BMS-906024*
- Camazepam
- Carburazepam
- Chlordiazepoxide
- Cinazepam
- Cinolazepam
- Clonazepam
- Cloniprazepam
- Clorazepate
- Cyprazepam
- Delorazepam
- Demoxepam
- Desmethylflunitrazepam
- Devazepide*
- Diazepam
- Diclazepam
- Difludiazepam
- Doxefazepam
- Elfazepam
- Ethyl carfluzepate
- Ethyl dirazepate
- Ethyl loflazepate
- Flubromazepam
- Fletazepam
- Fludiazepam
- Flunitrazepam
- Flurazepam
- Flutemazepam
- Flutoprazepam
- Fosazepam
- Gidazepam
- Halazepam
- Iclazepam
- Irazepine*
- Kenazepine
- Ketazolam
- Lorazepam
- Lormetazepam
- Lufuradom*
- Meclonazepam
- Medazepam
- Menitrazepam
- Metaclazepam
- Motrazepam
- N-Desalkylflurazepam
- Nifoxipam
- Nimetazepam
- Nitemazepam
- Nitrazepam
- Nitrazepate
- Nordazepam
- Nortetrazepam
- Oxazepam
- Phenazepam
- Pinazepam
- Pivoxazepam
- Prazepam
- Proflazepam
- Quazepam
- QH-II-66
- Reclazepam
- RO4491533*
- Ro05-4082
- Ro5-4864*
- Ro07-5220
- Ro07-9749
- Ro20-8065
- Ro20-8552
- SH-I-048A
- Sulazepam
- Temazepam
- Tetrazepam
- Tifluadom*
- Timelotem*
- Tolufazepam
- Triflunordazepam
- Tuclazepam
- Uldazepam
- Razobazam*
- Ripazepam
- Zolazepam
- Zomebazam
- Zometapine*
* atypical activity profile (not GABAA receptor ligands)










