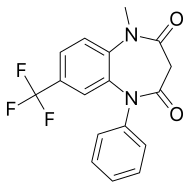
Triflubazam
Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| Chemical and physical data | |
| Formula | C17H13F3N2O2 |
| Molar mass | 334.298 g·mol−1 |
| 3D model (JSmol) |
|
| |
InChI
| |
| (verify) | |
Triflubazam[1] is a drug which is a 1,5-benzodiazepine derivative, related to clobazam.[2] It has sedative and anxiolytic effects, with a long half-life and duration of action.[3][4][5]
See also
- Benzodiazepine
- Clobazam
- CP-1414S
- Triflunordazepam
References
- ^ US 3660381, Karl-Heinz Weber KH, Merz H, K Zeile, Giesemann R, Danneberg P, "5-Aryl-1H-1,5-benzodiazepine-2,4-diones", issued 2 May 1972, assigned to CH Boehringer Sohn AG and Co KG
- ^ Lundbeck Institute (2003). "triflubazam". psychotropics.dk. Retrieved 17 November 2010.
- ^ Itil TM, Akpinar S, Fink M, Polvan N, Huque M, Sungurbey K (March 1976). "Controlled clinical and quantitative EEG studies of triflubazam (ORF 8063) in patients with anxiety syndrome". Current Therapeutic Research, Clinical and Experimental. 19 (3): 307–15. PMID 5248.
- ^ Csanalosi I, Pereira-Oran J, Case G, et al. (1977). "Triflubazam (ORF 8063), a new benzodiazepine in anxiety neurosis". Current Therapeutic Research. 22: 166–171.
- ^ Nicholson AN, Stone BM, Clarke CH (October 1977). "Effect of the 1,5-benzodiazepines, clobazam and triflubazam, on sleep in man". British Journal of Clinical Pharmacology. 4 (5): 567–72. doi:10.1111/j.1365-2125.1977.tb00787.x. PMC 1429140. PMID 20917.
- v
- t
- e
- 2-Oxoquazepam
- 3-Hydroxyphenazepam
- Bromazepam
- BMS-906024*
- Camazepam
- Carburazepam
- Chlordiazepoxide
- Cinazepam
- Cinolazepam
- Clonazepam
- Cloniprazepam
- Clorazepate
- Cyprazepam
- Delorazepam
- Demoxepam
- Desmethylflunitrazepam
- Devazepide*
- Diazepam
- Diclazepam
- Difludiazepam
- Doxefazepam
- Elfazepam
- Ethyl carfluzepate
- Ethyl dirazepate
- Ethyl loflazepate
- Flubromazepam
- Fletazepam
- Fludiazepam
- Flunitrazepam
- Flurazepam
- Flutemazepam
- Flutoprazepam
- Fosazepam
- Gidazepam
- Halazepam
- Iclazepam
- Irazepine*
- Kenazepine
- Ketazolam
- Lorazepam
- Lormetazepam
- Lufuradom*
- Meclonazepam
- Medazepam
- Menitrazepam
- Metaclazepam
- Motrazepam
- N-Desalkylflurazepam
- Nifoxipam
- Nimetazepam
- Nitemazepam
- Nitrazepam
- Nitrazepate
- Nordazepam
- Nortetrazepam
- Oxazepam
- Phenazepam
- Pinazepam
- Pivoxazepam
- Prazepam
- Proflazepam
- Quazepam
- QH-II-66
- Reclazepam
- RO4491533*
- Ro05-4082
- Ro5-4864*
- Ro07-5220
- Ro07-9749
- Ro20-8065
- Ro20-8552
- SH-I-048A
- Sulazepam
- Temazepam
- Tetrazepam
- Tifluadom*
- Timelotem*
- Tolufazepam
- Triflunordazepam
- Tuclazepam
- Uldazepam
- Arfendazam
- Clobazam
- CP-1414S
- Lofendazam
- Triflubazam
* atypical activity profile (not GABAA receptor ligands)
 | This sedative-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










