Bencyclane
 | |
 | |
| Names | |
|---|---|
| IUPAC name 3-[(1-benzylcycloheptyl)oxy]-N,N-dimethylpropan-1-amine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.016.861 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C19H31NO |
| Molar mass | 289.45554 |
| Pharmacology | |
| C04AX11 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Bencyclane is an antispasmodic, vasodilator, and platelet aggregation inhibitor.[1]
Synthesis
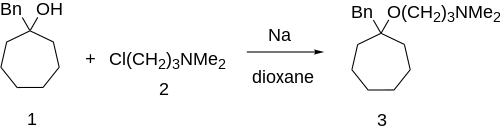
Grignard addition of benzylmagnesiumbromide to suberone would give 1-benzylcycloheptanol [4006-73-9] (1'). Williamson ether synthesis with 3-dimethylaminopropylchloride [109-54-6] (2) completed the synthesis of bencyclane (3).
See also
- Clofenciclan
References
- ^ J. Elks, ed. (2014). The Dictionary of Drugs: Chemical Data, Structures and Bibliographies. Springer.
- ^ Pallos, L.; Budai, Z.; Zólyomi, G. (1972). "Basic ethers of 1-substituted cycloalkanols. 1". Arzneimittel-Forschung. 22 (9): 1502–1505. PMID 4678613.
- ^ Pallos, L.; Budai, Z.; Zólyomi, G. (1972). "Basic ethers of 1-substituted cycloalkanols. 2". Arzneimittel-Forschung. 22 (9): 1505–1509. PMID 4678614.
- ^ Anon., ES 376120 (1972-04-01 to Gallardo Antonio SA).
- ^ Spaeter Genannt Werden Wird, DE 2455051A1 (1975 to Andreu S A Lab).
- v
- t
- e
Ion channel modulators
| VGKCsTooltip Voltage-gated potassium channels |
| ||||
|---|---|---|---|---|---|
| IRKsTooltip Inwardly rectifying potassium channel |
| ||||
| KCaTooltip Calcium-activated potassium channel |
| ||||
| K2PsTooltip Tandem pore domain potassium channel |
|
| VGSCsTooltip Voltage-gated sodium channels |
| ||||
|---|---|---|---|---|---|
| ENaCTooltip Epithelial sodium channel |
| ||||
| ASICsTooltip Acid-sensing ion channel |
|
| CaCCsTooltip Calcium-activated chloride channel |
| ||||
|---|---|---|---|---|---|
| CFTRTooltip Cystic fibrosis transmembrane conductance regulator |
| ||||
| Unsorted |
|
| TRPsTooltip Transient receptor potential channels |
|
|---|---|
| LGICsTooltip Ligand gated ion channels |
|
See also: Receptor/signaling modulators • Transient receptor potential channel modulators
 | This article about an amine is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










