WIN-2299
 | |
| Names | |
|---|---|
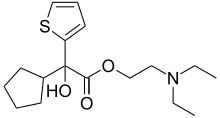
| Preferred IUPAC name 2-(Diethylamino)ethyl cyclopentyl(hydroxy)(thiophen-2-yl)acetate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.035.835 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C17H27NO3S |
| Molar mass | 325.47 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
WIN-2299 is an anticholinergic drug.[1] Human reactions to WIN-2299 include sedation (2 mg), LSD-like reactions (6 mg), and an acute delirious episode (10 mg).[2]
References
- ^ Luduena, FP; Lands, AM (1954). "An investigation of the pharmacological actions of three potent antispasmodic compounds and their corresponding metho-salts". The Journal of Pharmacology and Experimental Therapeutics. 110 (3): 282–92. PMID 13143475.
- ^ Pennes, HH; Hoch, PH (1957). "Psychotomimetics, clinical and theoretical considerations: Harmine, Win-2299 and nalline". The American Journal of Psychiatry. 113 (10): 887–92. doi:10.1176/ajp.113.10.887. PMID 13402982.
- v
- t
- e
Hallucinogens
(5-HT2A
agonists)
| Benzofurans |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyserg‐ amides |
| ||||||||||||||||||||||||||||||||||||||||
| Phenethyl‐ amines |
| ||||||||||||||||||||||||||||||||||||||||
| Piperazines | |||||||||||||||||||||||||||||||||||||||||
| Tryptamines |
| ||||||||||||||||||||||||||||||||||||||||
| Others |
|
(NMDAR
antagonists)
| Arylcyclo‐ hexylamines |
| ||||||
|---|---|---|---|---|---|---|---|
| Adamantanes | |||||||
| Diarylethylamines | |||||||
| Morphinans | |||||||
| Others |
(mAChR
antagonists)
- Atropine
- Benactyzine
- Benzatropine
- Benzydamine
- Biperiden
- BRN-1484501
- Brompheniramine
- BZ
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Chloropyramine
- Chlorphenamine
- Clemastine
- CS-27349
- Cyclizine
- Cyproheptadine
- Dicycloverine
- Dimenhydrinate
- Diphenhydramine
- Ditran
- Doxylamine
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Flavoxate
- Hyoscyamine
- JB-318
- JB-336
- Meclozine
- Mepyramine
- Orphenadrine
- Oxybutynin
- Pheniramine
- Phenyltoloxamine
- Procyclidine
- Promethazine
- Scopolamine
- Tolterodine
- Trihexyphenidyl
- Tripelennamine
- Triprolidine
- WIN-2299
 | This hallucinogen-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e













