Cilobamine
- none
- (2R,3R)-2-(3,4-Dichlorophenyl)-3-[(1-methylethyl)amino]bicyclo[2.2.2]octan-2-ol
- 69429-84-1
- 299379
- 8557262
- 067U1T4S30
- ChEMBL2106470
- Interactive image
- Clc1ccc(cc1Cl)[C@@]3(O)[C@H](NC(C)C)C2CCC3CC2
Cilobamine is a drug which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI) and has stimulant and antidepressant effects.[1][2]
It can clearly be seen that the structure is based on dichloroisoprenaline that has been fused onto the bicycloalkane scaffold.
Synthesis
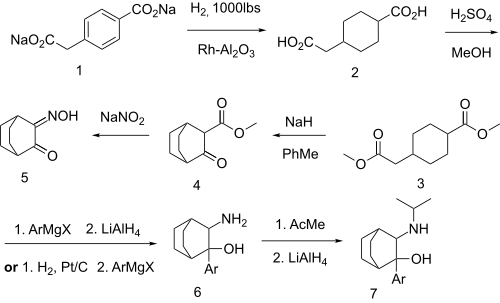
An intramolecular Dieckmann cyclization on methyl 4-(2-methoxy-2-oxoethyl)cyclohexanecarboxylate [1401222-79-4] (3) with sodium hydride base gives reaction Methyl 3-oxobicyclo[2.2.2]octane-2-carboxylate [30144-30-0] (4). Treatment with sodium nitrite introduces an isonitroso group adjacent to the ketone, giving 3-Hydroxyiminobicyclo[2.2.2]octan-2-one, CID:131066320 (5). Addition of the aryl Grignard reagent, and reduction of the oxime gives CID:154108204 (6). A reductive amination of the primary amino group with acetone then completed the synthesis of cilobamine (7).
See also
- Fencamfamine
- Manifaxine
References
- ^ Leeson GA, Shaath ZA, Biedenbach SA, Yarrington JT, Okerholm RA (April 1984). "Dose related induction of the drug metabolizing enzymes of rat liver by cilobamine". Fundamental and Applied Toxicology. 4 (2 Pt 1): 261–9. doi:10.1016/0272-0590(84)90127-1. PMID 6724198.
- ^ Wager S, Quitkin F, Stewart J, McGrath P, Harrison W, Markowitz J, Tricamo E (1988). "Cilobamine in the treatment of atypical depression". Human Psychopharmacology: Clinical and Experimental. 3 (3): 201–205. doi:10.1002/hup.470030308. S2CID 145253439.
- ^ DE2003744 idem Jules Freedman, U.S. patent 3,651,142 (1970 to Colgate Palmolive Co).
- v
- t
- e
- 3-Fluoromethcathinone
- 3,4-DMMC
- 4-BMC
- 4-CMC
- 4-Methylbuphedrone
- 4-Methylcathinone
- 4-MEAP
- 4-Methylpentedrone
- Amfepramone
- Benzedrone
- Buphedrone
- Bupropion
- Butylone
- Cathinone
- Dimethylcathinone
- Ethcathinone
- Ethylone
- Flephedrone
- Hexedrone
- Isoethcathinone
- Mephedrone
- Methcathinone
- Methedrone
- Methylenedioxycathinone
- Methylone
- Mexedrone
- N-Ethylbuphedrone
- N-Ethylhexedrone
- Pentedrone
- Pentylone
- Phthalimidopropiophenone
- A-84,543
- A-366,833
- ABT-202
- ABT-418
- AR-R17779
- Altinicline
- Anabasine
- Arecoline
- Bradanicline
- Cotinine
- Cytisine
- Dianicline
- Epibatidine
- Epiboxidine
- GTS-21
- Ispronicline
- Nicotine
- PHA-543,613
- PNU-120,596
- PNU-282,987
- Pozanicline
- Rivanicline
- Sazetidine A
- SIB-1553A
- SSR-180,711
- TC-1698
- TC-1827
- TC-2216
- Tebanicline
- UB-165
- Varenicline
- WAY-317,538
- 1-(4-Methylphenyl)-2-aminobutane
- 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane
- 2-Fluoroamphetamine
- 2-Fluoromethamphetamine
- 2-OH-PEA
- 2-Phenyl-3-aminobutane
- 2,3-MDA
- 3-Fluoroamphetamine
- 3-Fluoroethamphetamine
- 3-Methoxyamphetamine
- 3-Methylamphetamine
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-MA
- 4-MMA
- 4-MTA
- 6-FNE
- AL-1095
- Alfetamine
- a-Ethylphenethylamine
- Amfecloral
- Amfepentorex
- Amidephrine
- 2-Amino-1,2-dihydronaphthalene
- 2-Aminoindane
- 5-(2-Aminopropyl)indole
- 2-Aminotetralin
- Acridorex
- Amphetamine (Dextroamphetamine, Levoamphetamine)
- Amphetaminil
- Arbutamine
- β-Methylphenethylamine
- β-Phenylmethamphetamine
- Benfluorex
- Benzphetamine
- BDB
- BOH
- 3-Benzhydrylmorpholine
- BPAP
- Camfetamine
- Cathine
- Chlorphentermine
- Cilobamine
- Cinnamedrine
- Clenbuterol
- Clobenzorex
- Cloforex
- Clortermine
- Cypenamine
- D-Deprenyl
- Denopamine
- Dimethoxyamphetamine
- Dimethylamphetamine
- Dobutamine
- DOPA (Dextrodopa, Levodopa)
- Dopamine
- Dopexamine
- Droxidopa
- EBDB
- Ephedrine
- Epinephrine
- Epinine
- Etafedrine
- Ethylnorepinephrine
- Etilamfetamine
- Etilefrine
- Famprofazone
- Fencamfamin
- Fencamine
- Fenethylline
- Fenfluramine (Dexfenfluramine, Levofenfluramine)
- Fenproporex
- Feprosidnine
- Fludorex
- Formetorex
- Furfenorex
- Gepefrine
- Hexapradol
- HMMA
- Hordenine
- 4-Hydroxyamphetamine
- 5-Iodo-2-aminoindane
- Ibopamine
- Indanylamphetamine
- Iofetamine
- Isoetarine
- Isoprenaline
- L-Deprenyl (Selegiline)
- Lefetamine
- Lisdexamfetamine
- Lophophine
- MBDB
- MDA (tenamfetamine)
- MDBU
- MDEA
- MDMA (midomafetamine)
- MDMPEA
- MDOH
- MDPR
- MDPEA
- Mefenorex
- Mephentermine
- Metanephrine
- Metaraminol
- Mesocarb
- Methamphetamine (Dextromethamphetamine, Levomethamphetamine)
- Methoxamine
- Methoxyphenamine
- MMA
- Methoxyphenamine
- MMDA
- MMDMA
- MMMA
- Morforex
- N,alpha-Diethylphenylethylamine
- N,N-Dimethylphenethylamine
- Naphthylamphetamine
- Nisoxetine
- Norepinephrine
- Norfenefrine
- Norfenfluramine
- Normetanephrine
- L-Norpseudoephedrine
- Octopamine
- Orciprenaline
- Ortetamine
- Oxifentorex
- Oxilofrine
- PBA
- PCA
- PCMA
- PHA
- Pentorex
- Phenatine
- Phenpromethamine
- Phentermine
- Phenylalanine
- Phenylephrine
- Phenylpropanolamine
- Pholedrine
- PIA
- PMA
- PMEA
- PMMA
- PPAP
- Prenylamine
- Propylamphetamine
- Pseudoephedrine
- Ropinirole
- Salbutamol (Levosalbutamol)
- Sibutramine
- Solriamfetol
- Synephrine
- Theodrenaline
- Tiflorex
- Tranylcypromine
- Tyramine
- Tyrosine
- Xylopropamine
- Zylofuramine
- 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine
- 2-Benzylpiperidine
- 2-Methyl-3-phenylpiperidine
- 3,4-Dichloromethylphenidate
- 4-Benzylpiperidine
- 4-Fluoromethylphenidate
- 4-Methylmethylphenidate
- Desoxypipradrol
- Difemetorex
- Diphenylpyraline
- Ethylnaphthidate
- Ethylphenidate
- Methylnaphthidate
- Isopropylphenidate
- JZ-IV-10
- Methylphenidate (Dexmethylphenidate)
- Nocaine
- Phacetoperane
- Pipradrol
- Propylphenidate
- Serdexmethylphenidate
- SCH-5472
- 4-fluorotropacocaine
- 4'-Fluorococaine
- Altropane (IACFT)
- Brasofensine
- CFT (WIN 35,428)
- β-CIT (RTI-55)
- Cocaethylene
- Cocaine
- Dichloropane (RTI-111)
- Difluoropine
- FE-β-CPPIT
- FP-β-CPPIT
- Ioflupane (123I)
- Norcocaine
- PIT
- PTT
- RTI-31
- RTI-32
- RTI-51
- RTI-112
- RTI-113
- RTI-120
- RTI-121 (IPCIT)
- RTI-126
- RTI-150
- RTI-177
- RTI-229
- RTI-336
- RTI-354
- RTI-371
- RTI-386
- Salicylmethylecgonine
- Tesofensine
- Troparil (β-CPT, WIN 35,065-2)
- Tropoxane
- WF-23
- WF-33
- 2-MDP
- 3,3-Diphenylcyclobutanamine
- Amfonelic acid
- Amineptine
- Amiphenazole
- Atipamezole
- Atomoxetine
- Bemegride
- Benzydamine
- BTQ
- BTS 74,398
- Centanafadine
- Ciclazindol
- Clofenciclan
- Cropropamide
- Crotetamide
- D-161
- Desipramine
- Diclofensine
- Dimethocaine
- Efaroxan
- Etamivan
- Fenisorex
- Fenpentadiol
- Gamfexine
- Gilutensin
- GSK1360707F
- GYKI-52895
- Hexacyclonate
- Idazoxan
- Indanorex
- Indatraline
- JNJ-7925476
- Lazabemide
- Leptacline
- Lomevactone
- LR-5182
- Mazindol
- Meclofenoxate
- Medifoxamine
- Mefexamide
- Methamnetamine
- Methastyridone
- Methiopropamine
- Naphthylaminopropane
- Nefopam
- Nikethamide
- Nomifensine
- O-2172
- Oxaprotiline
- PNU-99,194
- PRC200-SS
- Rasagiline
- Rauwolscine
- Rubidium chloride
- Setazindol
- Tametraline
- Tandamine
- Thiopropamine
- Thiothinone
- Trazium
- UH-232
- Yohimbine
 | This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











