Lithium cyclopentadienide
 | |
| Names | |
|---|---|
| Other names lithium cyclopentadienylide, cyclopentadienyllithium, LiCp | |
| Identifiers | |
CAS Number |
|
| ECHA InfoCard | 100.156.001 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
Chemical formula | C5H5Li |
| Molar mass | 72.04 g·mol−1 |
| Appearance | colorless solid |
| Density | 1.064 g/cm3 |
Solubility in water | decomposition |
| Solubility | THF, dimethoxyethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Lithium cyclopentadienide is an organolithium compound with the formula C5H5Li. The compound is often abbreviated as LiCp, where Cp− is the cyclopentadienide anion. Lithium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
Preparation, structure and reactions
Lithium cyclopentadienide is commercially available as a solution in tetrahydrofuran. It is prepared by treating cyclopentadiene with butyllithium:[1]
- C5H6 + LiC4H9 → LiC5H5 + C4H10
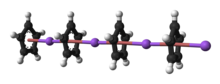
Because lithium cyclopentadienide is usually handled as a solution, the solvent-free solid is rarely encountered. According to X-ray crystallography, LiCp is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Li+ centers sandwiched between μ-η5:η5-C5H5 ligands.[2] In the presence of amines or ethers, LiCp gives adducts, e.g. (η5-Cp)Li(TMEDA).[1] LiCp is a common reagent for the preparation of cyclopentadienyl complexes.
See also
- Sodium cyclopentadienide
References
- ^ a b Reent Michel; Regine Herbst-Irmer; Dietmar Stalke (2011). "Revealing Coordination Patterns in C5-Cyclic Lithium Organics". Organometallics. 30 (16): 4379–4386. doi:10.1021/om200471e.
- ^ Robert E. Dinnebier; Ulrich Behrens & Falk Olbrich (1997). "Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. Determination by High-Resolution Powder Diffraction". Organometallics. 16 (17): 3855–3858. doi:10.1021/om9700122.
- v
- t
- e
- Li2
- LiAlCl4
- Li1+xAlxGe2−x(PO4)3
- LiAlH4
- LiAlO2
- LiAl1+xTi2−x(PO4)3
- LiAs
- LiAsF6
- Li3AsO4
- LiAt
- Li[AuCl4]
- LiB(C2O4)2
- LiB(C6F5)4
- LiBF4
- LiBH4
- LiBO2
- LiB3O5
- Li2B4O7
- Li2SnF6
- Li2TiF6
- Li2ZrF6
- Li2B4O7·5H2O
- LiBSi2
- LiBr
- LiBr·2H2O
- LiBrO
- LiBrO2
- LiBrO3
- LiBrO4
- Li2C2
- LiCF3SO3
- CH3CH(OH)COOLi
- LiC2H2ClO2
- LiC2H3IO2
- Li(CH3)2N
- LiCHO2
- LiCH3O
- LiC2H5O
- LiCN
- Li2CN2
- LiCNO
- Li2CO3
- Li2C2O4
- LiCl
- LiCl·H2O
- LiClO
- LiFO
- LiClO2
- LiClO3
- LiClO4
- LiCoO2
- Li2CrO4
- Li2CrO4·2H2O
- Li2Cr2O7
- CsLiB6O10
- LiD
- LiF
- Li2F
- LiF4Al
- Li3F6Al
- FLiBe
- LiFePO4
- FLiNaK
- LiGaH4
- Li2GeF6
- Li2GeO3
- LiGe2(PO4)3
- LiH
- LiH2AsO4
- Li2HAsO4
- LiHCO3
- Li3H(CO3)2
- LiH2PO3
- LiH2PO4
- LiHSO3
- LiHSO4
- LiHe
- LiI
- LiIO
- LiIO2
- LiIO3
- LiIO4
- Li2IrO3
- Li7La3Zr2O12
- LiMn2O4
- Li2MoO4
- Li0.9Mo6O17
- LiN3
- Li3N
- LiNH2
- Li2NH
- LiNO2
- LiNO3
- LiNO3·H2O
- Li2N2O2
- LiNa
- Li2NaPO3
- LiNaNO2
- LiNbO3
- Li2NbO3
- LiO−
- LiO2
- LiO3
- Li2O
- Li2O2
- LiOH
- Li3P
- LiPF6
- Li3PO4
- Li2HPO3
- Li2HPO4
- Li3PO3
- Li3PO4
- Li2Po
- Li2PtO3
- Li2RuO3
- Li2S
- LiSCN
- LiSH
- LiSO3F
- Li2SO3
- Li2SO4
- Li[SbF6]
- Li2Se
- Li2SeO3
- Li2SeO4
- LiSi
- Li2SiF6
- Li4SiO4
- Li2SiO3
- Li2Si2O5
- LiTaO3
- Li2Te
- LiTe3
- Li2TeO3
- Li2TeO4
- Li2TiO3
- Li4Ti5O12
- LiTi2(PO4)3
- LiVO3·2H2O
- Li3V2(PO4)3
- Li2WO4
- LiYF4
- LiZr2(PO4)3
- Li2ZrO3
- Hemolithin (extraterrestrial protein)
- Organolithium reagents
- CH3COOLi
- C4H6LiNO4
- LiC2F6NO4S2
- LiN(SiMe3)2
- Li3C6H5O7
- C5H5Li
- LiN(C3H7)2
- (C6H5)2PLi
- C18H35LiO3
- C6H13Li
- C4H9Li
- CH3CHLiCH2CH3
- (CH3)3CLi
- C12H28BLi
- CH3Li
- Li+C10H8−
- C5H11Li
- C5H3LiN2O4
- C6H5Li
- LiC2CH3
- LiO2C(CH2)16CH3
- C4H5LiO4
- LiEt3BH
- LiOC(CH3)3
- C9H18LiN
- LiC2H3 Vinyllithium
- LiC
11H
23COO
- Amblygonite
- Berezanskite
- Brannockite
- Cryolithionite
- Darapiosite
- Darrellhenryite
- Elbaite
- Fluorine Elbaite
- Fluor-liddicoatite
- Emeleusite
- Eucryptite LiAlSiO4
- Faizievite
- Hectorite
- Hsianghualite
- Jadarite LiNaSiB3O7OH
- Keatite Li(AlSi2O6)
- Kunzite
- Lavinskyite
- Lepidolite
- Lithiophilite LiMnPO4
- Lithiophosphate Li3PO4
- Manandonite
- Manganoneptunite
- Nambulite
- Neptunite
- Olympite
- Petalite LiAlSi4010
- Pezzottaite Cs(Be2Li)Al2Si6O18
- Rossmanite
- Saliotite
- Sogdianite
- Spodumene LiAl(SiO3)2
- Sugilite
- Tiptopite
- Tourmaline
- Triphylite LiFePO4
- Zabuyelite Li2CO3
- Zektzerite
- Zinnwaldite
- LixBey
- HLiHe+
- LiFHeO
- LiHe2
- (HeO)(LiF)2
- La2/3-xLi3xTiO3He
- Aluminium–lithium alloys
- Heteroatom-promoted lateral lithiation
- LB buffer
- Lithium atom
- Lithium medication
- LiNixCoyAlzO2
- LiNixMnyCozO2
- Lithium soap
- Lithium Triangle
- Lucifer yellow
- Magnesium–lithium alloys
- NASICON
- Environmentally friendly red light flare










