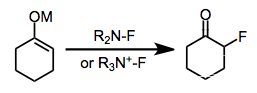
Electrophilic fluorination
Electrophilic fluorination is the combination of a carbon-centered nucleophile with an electrophilic source of fluorine to afford organofluorine compounds. Although elemental fluorine and reagents incorporating an oxygen-fluorine bond can be used for this purpose, they have largely been replaced by reagents containing a nitrogen-fluorine bond.[1]
Electrophilic fluorination offers an alternative to nucleophilic fluorination methods employing alkali or ammonium fluorides and methods employing sulfur fluorides for the preparation of organofluorine compounds. Development of electrophilic fluorination reagents has always focused on removing electron density from the atom attached to fluorine; however, compounds containing nitrogen-fluorine bonds have proven to be the most economical, stable, and safe electrophilic fluorinating agents. Electrophilic N-F reagents are either neutral or cationic and may possess either sp2- or sp3-hybridized nitrogen. Although the precise mechanism of electrophilic fluorination is currently unclear, highly efficient and stereoselective methods have been developed.
Some common fluorinating agents used for organic synthesis are N-fluoro-o-benzenedisulfonimide (NFOBS), N-fluorobenzenesulfonimide (NFSI), and Selectfluor.[1]
Mechanism and stereochemistry
Prevailing mechanism
The mechanism of electrophilic fluorination remains controversial. At issue is whether the reaction proceeds via an SN2 or single-electron transfer (SET) process. In support of the SN2 mechanism, aryl Grignard reagents and aryllithiums give similar yields of fluorobenzene in combination with N-fluoro-o-benzenedisulfonimide (NFOBS), even though the tendencies of these reagents to participate in SET processes differ substantially.[2] Additionally, radical probe experiments with 5-hexenyl and cyclopropyl enol ethers did not give any rearranged products.[3] More recently, kinetic studies on electrophilic fluorination of a series of 1,3-dicarbonyl derivatives by a range of N-F reagents have suggested the SN2 mechanism is more likely through Eyring and Hammett studies.[4]
On the other hand, the lifetime of radicals in the SET process is predicted to be four orders of magnitude shorter than the detection limit of even the most sensitive of radical probes. It has been postulated that after electron transfer, immediate recombination of the fluorine radical with the alkyl radical takes place.[5]
Stereoselective variants
Stereoselective fluorinations may be either diastereoselective or enantioselective. Diastereoselective methods have focused on the use of chiral auxiliaries on the nucleophilic substrate. For fluorinations of carbonyl compounds, chiral oxazolidinones have been used with success.[6]
Tandem conjugate addition incorporating a chiral nucleophile has been used to synthesize β-amino α-fluoro esters in chiral, non-racemic form.
Enantioselective methods employ stoichiometric amounts of chiral fluorinating agents. N-fluoroammonium salts of cinchona alkaloids represent the state of the art for reactions of this type. In addition, these reagents are easily synthesized from Selectfluor and the parent alkaloids.[7]
Scope and limitations
Fluorinating reagents
Electrophilic N-F fluorinating reagents incorporate electron-withdrawing groups attached to nitrogen to decrease the electron density on fluorine. Although N-fluorosulfonamides are fairly weak fluorinating reagents, N-fluorosulfonimides, such as N-fluorobenzenesulfonimide (NFSI), are very effective and in common use. N-fluoro-o-benzenedisulfonimide (NFOBS) is synthesized from the disulfonic acid.[2]
The use of salts of cationic nitrogen increases the rates and yields of electrophilic fluorination, because the cationic nitrogen removes electron density from fluorine. N-fluoropyridinium ions and iminium ions can also be used as electrophilic fluorinating reagents. The counteranions of these salts, although they are not directly involved in the transfer of fluorine to the substrate, influence reactivity in subtle ways and may be adjusted using a variety of methods.[8]
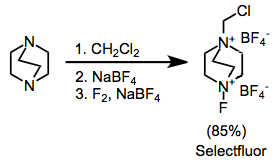
The most synthetically useful ammonium salts are the substituted DABCO bis(ammonium) ions, including Selectfluor.[9] These can be easily synthesized by alkylation followed by fluorination. The difluoro version, which might at first seem more useful, delivers only a single fluorine atom.
More specialized electrophilic fluorinating reagents, such as neutral heterocycles containing N–F bonds,[10] are useful for the fluorination of a limited range of substrates.
Nucleophilic substrates
Simple fluorinations of alkenes often produce complex mixtures of products. However, cofluorination in the presence of a nucleophile proceeds cleanly to give vicinal alkoxyfluorides.[11] Alkynes are not fluorinated with N-F reagents. An anionic surfactant was used to facilitate contact between aqueous Selectfluor and the alkene.
Fluorination of electron-rich aromatic compounds gives aryl fluorides. The two most common problems in this class of reactions are low ortho/para selectivities and dearomatization (the latter is a particularly significant problem for phenols).[12]
Enol ethers and glycals are nucleophilic enough to be fluorinated by Selectfluor.[13] Similar to other alkenes, cohalogenation can be accomplished either by isolation of the intermediate adduct and reaction with a nucleophile or direct displacement of DABCO in situ. Enols can be fluorinated enantioselectively (see above) in the presence of a chiral fluorinating agent.
Metal enolates are compatible with many fluorinating reagents, including NFSI, NFOBS, and sulfonamides. However, the specialized reagent 2-fluoro-3,3-dimethyl-2,3-dihydrobenzo[d]isothiazole 1,1-dioxide consistently affords better yields of monofluorinated carbonyl compounds in reactions with lithium enolates. Other metal enolates afforded large amounts of difluorinated products.[14]
Comparison with other methods
Although the use of molecular fluorine as an electrophilic fluorine source is often the cheapest and most direct method, F2 often forms radicals and reacts with C-H bonds without selectivity. Proton sources or Lewis acids are required to suppress radical formation, and even when these reagents are present, only certain substrates react with high selectivity.[15] Handling gaseous F2 requires extremely specialized and costly equipment.
Reagents containing O-F bonds, such as CF3OF, tend to be more selective for monofluorination than the N-F reagents.[16] However, difficulties associated with handling and their extreme oxidizing power have led to their replacement with N-F reagents.
Xenon di-, tetra-, and hexafluoride are selective monofluorinating reagents. However, their instability and high cost have made them less popular than the nitrogenous fluorinating agents.[17]
Typical conditions
Although fluorinations employing N-F reagents do not use molecular fluorine directly, they are almost universally prepared from F2. Proper handling of F2 requires great care and special apparatus.[18] Poly(tetrafluoroethylene) (PTFE, also known as Teflon) reaction vessels are used in preference to stainless steel or glass for reactions involving molecular fluorine. F2 blends with N2 or He are commercially available and help control the speed of delivery of fluorine. Temperatures should be kept low, and introduction of fluorine slow, to prevent free radical reactions.
See also
- Electrophilic halogenation
- Nucleophilic fluorination
References
- ^ a b Baudoux, Jérôme; Cahard, Dominique (2008). "Electrophilic Fluorination with <SCP>N</SCP> – <SCP>F</SCP> Reagents". Organic Reactions. pp. 1–326. doi:10.1002/0471264180.or069.02. ISBN 978-0-471-26418-7.
- ^ a b Davis, F. A.; Han, W.; Murphy, C. K. J. Org. Chem. 1995, 60, 4730.
- ^ Differding, E.; Rüegg, G. M. Tetrahedron Lett. 1991, 32, 3815.
- ^ Rozatian, Neshat; Ashworth, Ian W.; Sandford, Graham; Hodgson, David R.W. (2018). "A quantitative reactivity scale for electrophilic fluorinating reagents". Chemical Science. 9 (46): 8692–8702. doi:10.1039/C8SC03596B. PMC 6263395. PMID 30595834.
- ^ Piana, S.; Devillers, I.; Togni, A.; Rothlisberger, U. Angew. Chem. Int. Ed. Engl. 2002, 41, 979.
- ^ Davis, F. A.; Kasu, P. V. N. Tetrahedron Lett. 1998, 39, 6135.
- ^ Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001.
- ^ Umemoto, T.; Harasawa, K.; Tomizawa, G.; Kawada, K.; Tomita, K. Bull. Chem. Soc. Jpn. 1991, 64, 1081.
- ^ Stavber, S.; Zupan, M.; Poss, A. J.; Shia, G. A. Tetrahedron Lett. 1995, 36, 6769.
- ^ Laali, K. K.; Tanaka, M.; Forohar, F.; Cheng, M.; Fetzer, J. C. J. Fluorine Chem. 1998, 91, 185.
- ^ Lal, G. S. (1993). "Site-Selective Fluorination of Organic Compounds Using l-Alkyl-4-fluoro-l,4-diazabicyclo[2.2.2]octane Salts (Selectfluor Reagents)". J. Org. Chem. 58 (10): 2791. doi:10.1021/jo00062a023.
- ^ Zupan, M.; Iskra, J.; Stavber, S. (1995). "Chemistry of Organo Halogenic Molecules. 140. Role of the Reagent Structure on the Transformations of Hydroxy Substituted Organic Molecules with the N-Fluoro Class of Fluorinating Reagents". Bull. Chem. Soc. Jpn. 68 (6): 1655. doi:10.1246/bcsj.68.1655.
- ^ Albert, M.; Dax, K.; Ortner, J. Tetrahedron 1998, 54, 4839.
- ^ Differding, E.; Lang, R. W. Helv. Chim. Acta. 1989, 72, 1248.
- ^ Chambers, R. D.; Hutchinson, J.; Sandford, G. J. Fluorine Chem. 1999, 100, 63.
- ^ Rozen, S. Chem. Rev. 1996, 96, 1717.
- ^ Ramsden, C. A.; Smith, R. G. J. Am. Chem. Soc. 1998, 120, 6842.
- ^ Umemoto, T.; Nagayoshi, M. Bull. Chem. Soc. Jpn. 1996, 69, 2287.
- v
- t
- e
| HF | He | |||||||||||||||||
| LiF | BeF2 | BF BF3 B2F4 | CF4 CxFy | NF3 N2F4 | OF OF2 O2F2 O2F | F− | Ne | |||||||||||
| NaF | MgF2 | AlF AlF3 | SiF4 | P2F4 PF3 PF5 | S2F2 SF2 S2F4 SF4 S2F10 SF6 | ClF ClF3 ClF5 | HArF ArF2 | |||||||||||
| KF | CaF2 | ScF3 | TiF3 TiF4 | VF2 VF3 VF4 VF5 | CrF2 CrF3 CrF4 CrF5 CrF6 | MnF2 MnF3 MnF4 | FeF2 FeF3 | CoF2 CoF3 | NiF2 NiF3 | CuF CuF2 | ZnF2 | GaF3 | GeF4 | AsF3 AsF5 | SeF4 SeF6 | BrF BrF3 BrF5 | KrF2 KrF4 KrF6 | |
| RbF | SrF2 | YF3 | ZrF4 | NbF4 NbF5 | MoF4 MoF5 MoF6 | TcF6 | RuF3 RuF4 RuF5 RuF6 | RhF3 RhF5 RhF6 | PdF2 Pd[PdF6] PdF4 PdF6 | AgF AgF2 AgF3 Ag2F | CdF2 | InF3 | SnF2 SnF4 | SbF3 SbF5 | TeF4 TeF6 | IF IF3 IF5 IF7 | XeF2 XeF4 XeF6 XeF8 | |
| CsF | BaF2 | * | LuF3 | HfF4 | TaF5 | WF4 WF6 | ReF6 ReF7 | OsF4 OsF5 OsF6 OsF 7 OsF8 | IrF3 IrF5 IrF6 | PtF2 Pt[PtF6] PtF4 PtF5 PtF6 | AuF AuF3 Au2F10 AuF5·F2 | HgF2 Hg2F2 HgF4 | TlF TlF3 | PbF2 PbF4 | BiF3 BiF5 | PoF4 PoF6 | At | RnF2 RnF6 |
| Fr | RaF2 | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| ↓ | ||||||||||||||||||
| * | LaF3 | CeF3 CeF4 | PrF3 PrF4 | NdF3 | PmF3 | SmF2 SmF3 | EuF2 EuF3 | GdF3 | TbF3 TbF4 | DyF3 | HoF3 | ErF3 | TmF2 TmF3 | YbF2 YbF3 | ||||
| ** | AcF3 | ThF4 | PaF4 PaF5 | UF3 UF4 UF5 UF6 | NpF3 NpF4 NpF5 NpF6 | PuF3 PuF4 PuF5 PuF6 | AmF3 AmF4 AmF6 | CmF3 | Bk | Cf | Es | Fm | Md | No | ||||
- AgPF6
- KAsF6
- LiAsF6
- NaAsF6
- HPF6
- HSbF6
- NH4PF6
- KPF6
- KSbF6
- LiPF6
- NaPF6
- NaSbF6
- TlPF6
- Cs2AlF5
- K3AlF6
- Na3AlF6
and pseudohalogenides
- BaSiF6
- BaGeF6
- (NH4)2SiF6
- Na2[SiF6]
- K2[SiF6]
- CBrF3
- CBr2F2
- CBr3F
- CClF3
- CCl2F2
- CCl3F
- CF2O
- CF3I
- CHF3
- CH2F2
- CH3F
- C2Cl3F3
- C2H3F
- C6H5F
- C7H5F3
- C15F33N
- C3H5F
- C6H11F
lanthanide, actinide, ammonium
- VOF3
- CrOF4
- CrF2O2
- NH4F
- (NH4)2ZrF6
- CsXeF7
- Li2TiF6
- Li2ZrF6
- K2TiF6
- Rb2TiF6
- Na2TiF6
- Na2ZrF6
- K2NbF7
- K2TaF7
- K2ZrF6
- UO2F2
- FNO
- FNO2
- FNO3
- KHF2
- NaHF2
- NH4HF2
and iodosyl
- F2OS
- F3OP
- PSF3
- IOF3
- IO3F
- IOF5
- IO2F
- IO2F3