Mammalian protein found in Homo sapiens
| BMP1 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
3EDG, 3EDH |
|
|
| Identifiers |
|---|
| Aliases | BMP1, bone morphogenetic protein 1, OI13, PCOLC, PCP, PCP2, TLD |
|---|
| External IDs | OMIM: 112264; MGI: 88176; HomoloGene: 55955; GeneCards: BMP1; OMA:BMP1 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 8 (human)[1] |
|---|
| | Band | 8p21.3 | Start | 22,165,140 bp[1] |
|---|
| End | 22,212,326 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 14 (mouse)[2] |
|---|
| | Band | 14 D2|14 36.32 cM | Start | 70,474,558 bp[2] |
|---|
| End | 70,520,234 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - stromal cell of endometrium
- left uterine tube
- right adrenal cortex
- left coronary artery
- canal of the cervix
- ectocervix
- left adrenal cortex
- right coronary artery
- body of uterus
- gastric mucosa
|
| | Top expressed in | - gastrula
- cerebellar cortex
- stroma of bone marrow
- lip
- decidua
- body of femur
- external carotid artery
- internal carotid artery
- calvaria
- molar
|
| | More reference expression data |
|
|---|
| BioGPS | 

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - calcium ion binding
- zinc ion binding
- metal ion binding
- peptidase activity
- protein binding
- metalloendopeptidase activity
- growth factor activity
- hydrolase activity
- metallopeptidase activity
- serine-type endopeptidase activity
- cytokine activity
- identical protein binding
| | Cellular component | - Golgi apparatus
- extracellular region
- vesicle
- extracellular space
| | Biological process | - skeletal system development
- cell differentiation
- ossification
- extracellular matrix disassembly
- proteolysis
- multicellular organism development
- cartilage development
- positive regulation of cartilage development
- cartilage condensation
- high-density lipoprotein particle assembly
- regulation of signaling receptor activity
- signal transduction
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | NM_001199
NM_006128
NM_006129
NM_006130
NM_006131
|
|---|
NM_006132 |
| |
|---|
| RefSeq (protein) | | |
|---|
| Location (UCSC) | Chr 8: 22.17 – 22.21 Mb | Chr 14: 70.47 – 70.52 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Bone morphogenetic protein 1, also known as BMP1, is a protein which in humans is encoded by the BMP1 gene.[5][6] There are seven isoforms of the protein created by alternate splicing.
Function
BMP1 belongs to the peptidase M12A family of bone morphogenetic proteins (BMPs). It induces bone and cartilage development. Unlike other BMPs, it does not belong to the TGFβ superfamily. It was initially discovered to work like other BMPs by inducing bone and cartilage development. It however, is a metalloprotease that cleaves the C-terminus of procollagen I, II and III. It has an astacin-like protease domain.
It has been shown to cleave laminin 5 and is localized in the basal epithelial layer of bovine skin.
The BMP1 locus encodes a protein that is capable of inducing formation of cartilage in vivo. Although other bone morphogenetic proteins are members of the TGF-beta superfamily, BMP1 encodes a protein that is not closely related to other known growth factors. BMP1 protein and procollagen C proteinase (PCP), a secreted metalloprotease requiring calcium and needed for cartilage and bone formation, are identical. PCP or BMP1 protein cleaves the C-terminal propeptides of procollagen I, II, and III and its activity is increased by the procollagen C-endopeptidase enhancer protein. The BMP1 gene is expressed as alternatively spliced variants that share an N-terminal protease domain but differ in their C-terminal region[5]
Structure
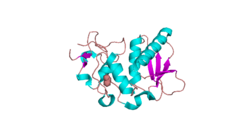
A partial structure of BMP1 was determined through X-Ray diffraction with a resolution of 1.27 Å.[7] Crystallization experiments were done by vapor diffusion at a pH of 7.5. This is important because it is close to the pH of the human body, where BMP1 resides in vivo. This BMP1 fragment is 202 residues in length. Its secondary structure is made up of 30% helices, or 10 helices, 61 residues in length, and 15% beta sheets, or 11 strands, 32 residues in length. It contains ligands of an acetyl group and a Zinc ion.
A Ramachandran plot was constructed for BMP1.[8] This plot shows that BMP1 most prefers Phi and Psi angles (Phi, Psi) of around (-60°,-45°) and (-60°, 140°). These preferred angles are an estimate of the most clustered data of the Ramachandran plot. The preferred region is much greater in range. 97% of the residues were in preferred regions and 100% of the residues were in the allowed region, with no outliers.
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000168487 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000022098 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b "Entrez Gene: BMP1 bone morphogenetic protein 1".
- ^ Tabas JA, Zasloff M, Wasmuth JJ, Emanuel BS, Altherr MR, McPherson JD, Wozney JM, Kaplan FS (February 1991). "Bone morphogenetic protein: chromosomal localization of human genes for BMP1, BMP2A, and BMP3". Genomics. 9 (2): 283–9. doi:10.1016/0888-7543(91)90254-C. PMID 2004778.
- ^ PDB: 3EDG; Mac Sweeney A, Gil-Parrado S, Vinzenz D, Bernardi A, Hein A, Bodendorf U, Erbel P, Logel C, Gerhartz B (December 2008). "Structural basis for the substrate specificity of bone morphogenetic protein 1/tolloid-like metalloproteases". J. Mol. Biol. 384 (1): 228–39. doi:10.1016/j.jmb.2008.09.029. PMID 18824173.
- ^ "MolProbity Ramachandran analysis of 3EDG, model 1" (PDF). www.rcsb.org. Archived from the original (PDF) on 2012-10-12.
Further reading
- Tabas JA, Zasloff M, Wasmuth JJ, et al. (1991). "Bone morphogenetic protein: chromosomal localization of human genes for BMP1, BMP2A, and BMP3". Genomics. 9 (2): 283–9. doi:10.1016/0888-7543(91)90254-C. PMID 2004778.
- Wozney JM, Rosen V, Celeste AJ, et al. (1989). "Novel regulators of bone formation: molecular clones and activities". Science. 242 (4885): 1528–34. doi:10.1126/science.3201241. PMID 3201241.
- Takahara K, Lyons GE, Greenspan DS (1995). "Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues". J. Biol. Chem. 269 (51): 32572–8. doi:10.1016/S0021-9258(18)31672-7. PMID 7798260.
- Yoshiura K, Tamura T, Hong HS, et al. (1993). "Mapping of the bone morphogenetic protein 1 gene (BMP1) to 8p21: removal of BMP1 from candidacy for the bone disorder in Langer-Giedion syndrome". Cytogenet. Cell Genet. 64 (3–4): 208–9. doi:10.1159/000133577. PMID 8404039.
- Takahara K, Lee S, Wood S, Greenspan DS (1996). "Structural organization and genetic localization of the human bone morphogenetic protein 1/mammalian tolloid gene". Genomics. 29 (1): 9–15. doi:10.1006/geno.1995.1209. PMID 8530106.
- Kessler E, Takahara K, Biniaminov L, et al. (1996). "Bone morphogenetic protein-1: the type I procollagen C-proteinase". Science. 271 (5247): 360–2. Bibcode:1996Sci...271..360K. doi:10.1126/science.271.5247.360. PMID 8553073. S2CID 26378870.
- Li SW, Sieron AL, Fertala A, et al. (1996). "The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1". Proc. Natl. Acad. Sci. U.S.A. 93 (10): 5127–30. Bibcode:1996PNAS...93.5127L. doi:10.1073/pnas.93.10.5127. PMC 39418. PMID 8643539.
- Janitz M, Heiser V, Böttcher U, et al. (1998). "Three alternatively spliced variants of the gene coding for the human bone morphogenetic protein-1". J. Mol. Med. 76 (2): 141–6. doi:10.1007/s001090050202. PMID 9500680. S2CID 26374071.
- Scott IC, Blitz IL, Pappano WN, et al. (1999). "Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis". Dev. Biol. 213 (2): 283–300. doi:10.1006/dbio.1999.9383. PMID 10479448.
- Amano S, Scott IC, Takahara K, et al. (2000). "Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain". J. Biol. Chem. 275 (30): 22728–35. doi:10.1074/jbc.M002345200. PMID 10806203.
- Scott IC, Blitz IL, Pappano WN, et al. (2001). "Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling". Nature. 410 (6827): 475–8. Bibcode:2001Natur.410..475S. doi:10.1038/35068572. PMID 11260715. S2CID 4392365.
- Garrigue-Antar L, Barker C, Kadler KE (2001). "Identification of amino acid residues in bone morphogenetic protein-1 important for procollagen C-proteinase activity". J. Biol. Chem. 276 (28): 26237–42. doi:10.1074/jbc.M010814200. PMID 11283002.
- Unsöld C, Pappano WN, Imamura Y, et al. (2002). "Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases". J. Biol. Chem. 277 (7): 5596–602. doi:10.1074/jbc.M110003200. PMID 11741999.
- Rattenholl A, Pappano WN, Koch M, et al. (2002). "Proteinases of the bone morphogenetic protein-1 family convert procollagen VII to mature anchoring fibril collagen". J. Biol. Chem. 277 (29): 26372–8. doi:10.1074/jbc.M203247200. PMID 11986329.
- Garrigue-Antar L, Hartigan N, Kadler KE (2003). "Post-translational modification of bone morphogenetic protein-1 is required for secretion and stability of the protein". J. Biol. Chem. 277 (45): 43327–34. doi:10.1074/jbc.M207342200. PMID 12218058.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Hartigan N, Garrigue-Antar L, Kadler KE (2003). "Bone morphogenetic protein-1 (BMP-1). Identification of the minimal domain structure for procollagen C-proteinase activity". J. Biol. Chem. 278 (20): 18045–9. doi:10.1074/jbc.M211448200. PMID 12637537.
- Leighton M, Kadler KE (2003). "Paired basic/Furin-like proprotein convertase cleavage of Pro-BMP-1 in the trans-Golgi network". J. Biol. Chem. 278 (20): 18478–84. doi:10.1074/jbc.M213021200. PMID 12637569.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Hillman RT, Green RE, Brenner SE (2005). "An unappreciated role for RNA surveillance". Genome Biol. 5 (2): R8. doi:10.1186/gb-2004-5-2-r8. PMC 395752. PMID 14759258.
External links
- bone+morphogenetic+protein+1 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human BMP1 genome location and BMP1 gene details page in the UCSC Genome Browser.
- Human PCP2 genome location and PCP2 gene details page in the UCSC Genome Browser.
- The MEROPS online database for peptidases and their inhibitors: M12.005[permanent dead link]





















