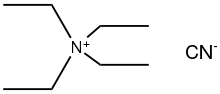
Tetraethylammonium cyanide
 | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.033.228 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C9H20N2 |
| Molar mass | 156.273 g·mol−1 |
| Appearance | white solid |
| Melting point | 254 °C (489 °F; 527 K) |
| Hazards | |
| GHS labelling: | |
Pictograms |   |
| Danger | |
Hazard statements | H300, H310, H330, H410 |
Precautionary statements | P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 |
| Related compounds | |
Other anions | Tetraethylammonium chloride Tetraethylammonium bromide Tetraethylammonium iodide |
Other cations | Tetramethylammonium cyanide Ammonium cyanide Guanidinium cyanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Tetraethylammonium cyanide is the organic compound with the formula (C2H5)4NCN. It is a "quat salt" of cyanide. It is a colorless, deliquescent solid that is soluble in polar organic media. It is used in the synthesis of cyanometallates.[1]
Tetraethylammonium cyanide is prepared by ion exchange from tetraethylammonium bromide. The corresponding tetraphenylarsonium salt is prepared similarly.[2]
Safety
The salt is highly toxic.
See also
References
- ^ Entley, William R.; Treadway, Christopher R.; Wilson, Scott R.; Girolami, Gregory S. (1997). "The Hexacyanotitanate Ion: Synthesis and Crystal Structure of [NEt4]3[TiIII(CN)6]·4MeCN". Journal of the American Chemical Society. 119 (27): 6251–6258. doi:10.1021/ja962773m.
- ^ Dieck, R. L.; Peterson, E. J.; Galliart, A.; Brown, T. M.; Moeller, T. (1976). "Tetraethylammonium, Tetraphenylarsonium, and Ammonium Cyanates and Cyanides". Inorganic Syntheses. Vol. 16. pp. 131–137. doi:10.1002/9780470132470.ch36. ISBN 9780470132470.












