Colestipol
- C10AC02 (WHO)
- US: ℞-only
- Copolymer of bis(2-aminoethyl)amine and 2-(chloromethyl)oxirane
- 26658-42-4
 N
N
37296-80-3 (HCl)
- 62816
- DB00375
 Y
Y
- none
- K50N755924
- C06925
 Y
Y
- ChEMBL1201678
 Y
Y
- DTXSID00965206 DTXSID901009549, DTXSID00965206

 N
N Y (what is this?) (verify)
Y (what is this?) (verify)Colestipol (trade names Colestid, Cholestabyl) is a bile acid sequestrant used to lower blood cholesterol, specifically low-density lipoprotein (LDL).[1][2] It is also used to reduce stool volume and frequency, and in the treatment of chronic diarrhea.[3]
Like cholestyramine, colestipol works in the gut by trapping bile acids and preventing them from being reabsorbed. This leads to decreased enterohepatic recirculation of bile acids, increased synthesis of new bile acids by the liver from cholesterol, decreased liver cholesterol, increased LDL receptor expression, and decreasing LDL in blood.[4]
Side effects
The following notable side effects may occur:[2]
- gastrointestinal tract disturbances, especially (mild, occasionally severe) constipation
- sometimes increase in VLDL[citation needed] and triglyceride synthesis
Interactions
Colestipol can bind to a number of drugs and nutrients in the gut and inhibit or delay their absorption. Such substances include:[2]
- thiazide diuretics, furosemide
- gemfibrozil
- benzylpenicillin, tetracycline
- digoxin
- lipid-soluble vitamins (A, D, E, K)
Contraindications
Colestipol is contraindicated in hypertriglyceridemia (high level of triglycerides in the blood).[citation needed]
Chemistry
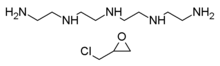
Colestipol is a copolymer of diethylenetriamine (DETA) —or tetraethylenepentamine according to some sources[5][6]— and epichlorohydrin.[7][8] The structure drawing (top right) shows the DETA moieties in blue and the epichlorohydrin moieties in red.
 |   |
Notes and references
- ^ Handelsman Y (May 2011). "Role of bile acid sequestrants in the treatment of type 2 diabetes". Diabetes Care. 34 (Suppl 2): S244-50. doi:10.2337/dc11-s237. PMC 3632187. PMID 21525463.
- ^ a b c "Colestipol Hydrochloride". Drugs.com.
- ^ "colestipol (Colestid)". MedicineNet.
- ^ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 523. ISBN 3-8047-1763-2.
- ^ "Colestipol structure". Clinical Pharmacology. Archived from the original on 2016-03-04. Retrieved 2012-01-28.
- ^ "Colestipol structure". Beth Israel Deaconess Medical Center & Care Group. Archived from the original on 2010-12-29.
- ^ Haberfeld H, ed. (2009). Austria-Codex (in German) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- ^ Steinhilber D, Schubert-Zsilavecz M, Roth HJ (2005). Medizinische Chemie (in German). Stuttgart: Deutscher Apotheker Verlag. p. 433. ISBN 3-7692-3483-9.
- v
- t
- e
| Cholesterol absorption inhibitors, NPC1L1 | |
|---|---|
| Bile acid sequestrants/resins (LDL) |
|
| Statins (HMG-CoA reductase, LDL) | |
|---|---|
| Niacin and derivatives (HDL and LDL) | |
| MTTP inhibitors (VLDL) | |
| ATP citrate lyase inhibitors (LDL) | |
| Thyromimetics (VLDL) |
| PPAR agonists (LDL) |
| ||||
|---|---|---|---|---|---|
| CETP inhibitors (HDL) | |||||
| PCSK9 inhibitors (LDL) | |||||
| ANGPTL3 inhibitors (LDL/HDL) |
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III












