Amaninamide
 | |
 | |
| Names | |
|---|---|
| Other names 4-(2-mercapto-L-tryptophan)-alpha-Amanitin | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
InChI
| |
| |
| Properties | |
Chemical formula | C39H54N10O13S |
| Molar mass | 902.97 g/mol |
| Appearance | Colorless, crystalline solid |
Solubility in water | Soluble |
| Solubility in ethanol, methanol | Soluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
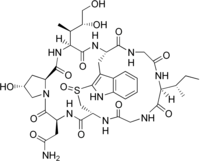
Amaninamide is a cyclic peptide. It is one of the amatoxins, all of which are found in several members of the mushroom genera Amanita, Lepiota and Galerina. It differs from alpha-amanitin in lacking the hydroxyl group on tryptophan. This alters its UV absorption spectrum but not its toxicity.
Toxicology
Like other amatoxins, amaninamide is an inhibitor of RNA polymerase II. Upon ingestion, it binds to the RNA polymerase II enzyme which completely prevents mRNA synthesis, effectively causing cytolysis of hepatocytes (liver cells) and kidney cells.[1]
See also
References
- ^ M. Cochet-Meillhac; Chambon P. (1974). "Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins". Biochim Biophys Acta. 353 (2): 160–184. doi:10.1016/0005-2787(74)90182-8. PMID 4601749.
External links
- Amatoxins REVISED Archived 2009-01-13 at the Wayback Machine
- Poisonous Mushrooms (German)
- v
- t
- e
Poisonous Amanita mushrooms
Amanita
| Amanita |
|
|---|

Amanitina
| Lepidella (=Saproamanita) |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phalloideae |
| ||||||||||||||||
| Roanokenses |
| ||||||||||||||||
| Validae |
|











