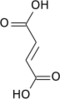
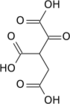
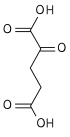
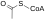Aconitic acid
 cis-aconitic acid | |
 trans-aconitic acid | |
| Names | |
|---|---|
| Preferred IUPAC name Prop-1-ene-1,2,3-tricarboxylic acid | |
| Other names Achilleic acid; equisetic acid; citridinic acid; pyrocitric acid; achilleaic acid; acinitic acid | |
| Identifiers | |
CAS Number |
|
| ChemSpider |
|
| ECHA InfoCard | 100.007.162 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| Properties | |
Chemical formula | C6H6O6 |
| Molar mass | 174.108 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) (mixed isomers), 173 °C (cis and trans isomers) |
| Acidity (pKa) | 2.80, 4.46 (trans isomer)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
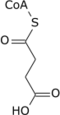
Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid:[3]
- (HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O
A mixture of isomers are generated in this way.
Aconitic acid was originally isolated from Aconitum napellus by Swiss chemist and apothecary Jacques Peschier in 1820.[4][5] It was first prepared by thermal dehydration.[6]
References
- ^ "Aconitic Acid - Compound Summary (CID 309)". PubChem.
- ^ Dawson, R. M. C.; Elliott, D. C.; Elliott, W. H. (1989). Data for Biochemical Research (3rd ed.). Oxford: Clarendon Press. ISBN 9780198552994.
- ^ Bruce, W. F. (1937). "Aconitic Acid". Organic Syntheses. 17: 1. doi:10.15227/orgsyn.017.0001.
- ^ Brande, William Thomas (1848). A Manual of Chemistry, Vol II (6 ed.). London: John W. Parker. p. 1344. Retrieved 8 November 2023.
- ^ Reichenbach, Karl-Rudolf (2001). Jacques Peschier (1769-1832): Ein Genfer Apotheker und Chemiker. Zürich: Wissenschaftliche Verlagsgesellschaft mbH Stuttgart. ISBN 3804719090. Retrieved 8 November 2023.
- ^ Pawolleck, B. (1875). "Substitutionsproducte der Citronensäure und ein Versuch zur Synthese der letzteren" [Substitution products of citric acid and an attempt at the synthesis of the latter]. Justus Liebig's Annalen der Chemie. 178 (2–3): 150–170. doi:10.1002/jlac.18751780203.
- v
- t
- e
| + H2O |  NADH +H+ NAD+  H2O  FADH2 FAD  CoA + ATP (GTP) Pi + ADP (GDP) | |
 |  | NADH + H+ + CO2 |
| CoA | NAD+ | |