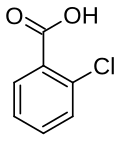
2-Chlorobenzoic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2-Chlorobenzoic acid | |
| Other names o-Chlorobenzoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.003.897 |
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C7H5ClO2 |
| Molar mass | 156.57 g·mol−1 |
| Appearance | white solid |
| Melting point | 142 °C (288 °F; 415 K) |
| Boiling point | 285 °C (545 °F; 558 K) |
| log P | 2.039 [1] |
| Acidity (pKa) | 2.89 [2] [3] |
Magnetic susceptibility (χ) | -83.56·10−6 cm3/mol |
| Hazards | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
2-Chlorobenzoic acid is an organic compound with the formula ClC6H4CO2H. It is one of three isomeric chlorobenzoic acids, the one that is the strongest acid. This white solid is used as a precursor to a variety of drugs, food additives, and dyes.[4]
Synthesis and reactions
It is prepared by the oxidation of 2-chlorotoluene. The laboratory scale reaction employs potassium permanganate.[5] Alternatively it arises by the hydrolysis of α,α,α-trichloro-2-toluene.
The chloride is readily replaced by ammonia to 2-aminobenzoic acid. Similarly, the chloride is displaced by diphenylphosphide, leading to 2-diphenylphosphinobenzoic acid.
At elevated temperature it decarboxylates.[4]
References
- ^ 1.2-Chlorobenzoic acid; C7H5ClO2; ChemSpider. Chemspider.com (2015). at [1]
- ^ Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 1838
- ^ 2.2-Chlorobenzoic acid; C7H5ClO2 - PubChem. Pubchem.ncbi.nlm.nih.gov at [2]
- ^ a b Takao Maki, Kazuo Takeda "Benzoic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_555.
- ^ H. T. Clarke and E. R. Taylor (1943). "o-Chlorobenzoic acid". Organic Syntheses; Collected Volumes, vol. 2, p. 135.
- v
- t
- e












